A comparative study of the photophysics of phenyl, thienyl, and chalcogen substituted rhodamine dyes†
Abstract
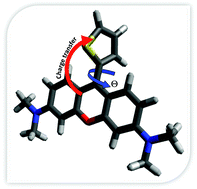
Although rhodamine dyes have been extensively studied for a variety of applications, many details of their photophysics are not yet fully understood, including the possible presence of a charge separated electronic state lying near the optically active excited singlet state and the role of twisting substituent groups in excited-state quenching. To address this, a large library of rhodamine dyes was studied in which the chalcogen is varied from O, to S and Se and the aryl group is either absent (in the pyronin series) or is a phenyl or thienyl substituent. Through an analysis of steady-state absorption spectroscopy, electrochemistry, X-ray crystallography, and quantum mechanical calculations, we show that the lowest unoccupied molecular orbital (LUMO) energy decreases in the O → S → Se series and when a phenyl or thienyl substituent is added. The reduction of the LUMO energy is larger for thienyl species in which the aromatic group has increased torsional flexibility. Excited state lifetimes and fluorescence quantum yields of these dyes in a high and low polarity solvent reveal dramatically different photophysics between chromophores with phenyl and thienyl substituents, due to a combination of torsional and inductive effects. In the pyronin and phenyl-substituted species, non-radiative decay can occur through an amine-to-xanthylium core charge separated state that is stabilized in a highly polar environment. In the thienyl derivatives, a lower energy excited state, which we term S′1, is accessed from S1via rotation of the aryl group and the excited state population rapidly equilibrates between S1 and S′1 in 6–30 ps. Preliminary photochemical hydrogen production data display the potential application of the thienyl derivatives for conversion of solar energy.

 Please wait while we load your content...
Please wait while we load your content...