The internal heavy-atom effect on 3-phenylselanyl and 3-phenyltellanyl BODIPY derivatives studied by transient absorption spectroscopy†
Abstract
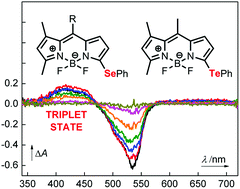
Three monosubstituted 3-phenylselanyl and 3-phenyltellanyl BODIPY derivatives were synthesized and their spectroscopic properties were characterized and compared to those of iodine and chlorine-atoms containing analogues as well as an unsubstituted BODIPY derivative. The fluorescence quantum yields were found to decrease, whereas the intersystem crossing quantum yields (ΦISC), determined by transient spectroscopy, increased in the order of the H → Cl → Se/I → Te substitution. The maximum ΦISC, found for the 3-phenyltellanyl derivative, was 59%. The results are interpreted in terms of the internal heavy-atom effect of the substituents.

 Please wait while we load your content...
Please wait while we load your content...