Twisting the ethano-Tröger's base: the bisamide†
Abstract
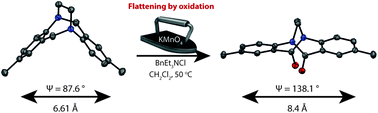
The typically planar amide when incorporated into bicyclic systems can undergo a significant distortion from planarity resulting in physical properties and reactivity that deviate from classical amide behaviour. Herein, we report a succinct protocol that utilises potassium permanganate to selectively α-oxygenate the benzylic position of ethano-Tröger's base derivatives to yield a new class of twisted bisamides. Additionally, we report the first synthesis of an ethano-Tröger's base derivative bearing substituents in the positions ortho to the nitrogen atoms.
- This article is part of the themed collection: Celebrating excellence in research: women of organic chemistry


 Please wait while we load your content...
Please wait while we load your content...