5-Aryl-2-(naphtha-1-yl)sulfonamido-thiazol-4(5H)-ones as clathrin inhibitors†
Abstract
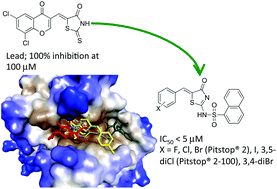
The development of a (Z)-5-((6,8-dichloro-4-oxo-4H-chromen-3-yl)methylene)-2-thioxothiazolidin-4-one (2), rhodanine-based lead that led to the Pitstop® 2 family of clathrin inhibitors is described herein. Head group substitution and bioisosteric replacement of the rhodanine core with a 2-aminothiazol-4(5H)-one scaffold eliminated off target dynamin activity. A series of N-substituents gave first phenylglycine (20, IC50 ∼ 20 μM) then phenyl (25, IC50 ∼ 7.1 μM) and 1-napthyl sulfonamide (26, Pitstop® 2 compound, IC50 ∼ 1.9 μM) analogues with good activity, validating this approach. A final library exploring the head group resulted in three analogues displaying either slight improvements or comparable activity (33, 38, and 29 with IC50 ∼ 1.4, 1.6 and 1.8 μM respectively) and nine others with IC50 < 10 μM. These results were rationalized using in silico docking studies. Docking studies predicted enhanced Pitstop® 2 family binding, not a loss of binding, within the Pistop® groove of the reported clathrin mutant invalidating recent assumptions of poor selectivity for this family of clathrin inhibitors.

 Please wait while we load your content...
Please wait while we load your content...