Catalytic enantioselective cascade Michael/cyclization reaction of 3-isothiocyanato oxindoles with exocyclic α,β-unsaturated ketones en route to 3,2′-pyrrolidinyl bispirooxindoles†
Abstract
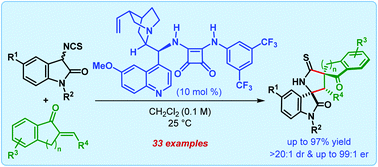
Cascade Michael/cyclization reactions between 3-isothiocyanato oxindoles and exocyclic α,β-unsaturated ketones are shown to proceed efficiently in the presence of a quinine-derived tertiary amino-squaramide catalyst and furnish 3,2′-pyrrolidinyl bispirooxindoles containing two spiro-quaternary and three contiguous stereocenters as a single diastereomer with excellent enantioselectivities (up to 99 : 1 er).


 Please wait while we load your content...
Please wait while we load your content...