Synthesis of quinazolines from 2-aminobenzylamines with benzylamines and N-substituted benzylamines under transition metal-free conditions†
Abstract
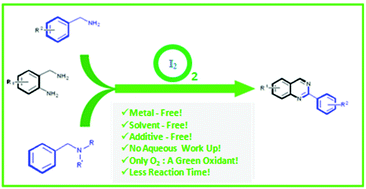
This work reports the synthesis of quinazolines from 2-aminobenzylamines with N-substituted benzylamines in the presence of molecular iodine. The developed methodology works smoothly under transition-metal free, additive free and solvent free conditions. The use of O2 as a green oxidant makes it a greatly economical, green and sustainable protocol. Moreover, no aqueous work up is required thereby enhancing the efficiency. A series of quinazoline derivatives were synthesized successfully in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...