Silver-catalyzed nucleophilic substitution of aminals with ethyl diazoacetate: a new pathway to β-amino-α-diazoesters†
Abstract
A novel silver-catalyzed nucleophilic substitution of aminals with ethyl diazoacetate producing β-amino-α-diazoesters has been developed. A series of aminals with different amino moieties and substituents were successfully incorporated in this reaction, which delivered a wide range of β-amino-α-diazocarbonyl compounds in the presence of a lower catalyst loading in 2 hours.
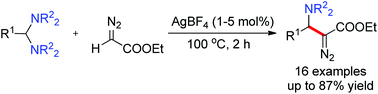

 Please wait while we load your content...
Please wait while we load your content...