BF3·Et2O mediated one-step synthesis of N-substituted-1,2-dihydropyridines, indenopyridines and 5,6-dihydroisoquinolines†
Abstract
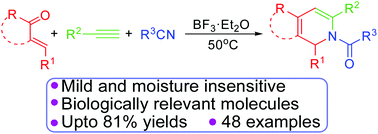
A simple and efficient one-pot synthesis of N-substituted-1,2-dihydropyridines, indenopyridines and 5,6-dihydroisoquinolines by a BF3·Et2O mediated novel methodology, from easily available α,β-unsaturated ketones/arylidene ketones, phenyl acetylenes and substituted nitriles, has been described. This novel annulation provides quick access to complex polycyclic frameworks with an excellent substrate scope.


 Please wait while we load your content...
Please wait while we load your content...