Gold(i)-catalyzed addition of aldehydes to cyclopropylidene bearing 6-aryl-1,5-enynes†
Abstract
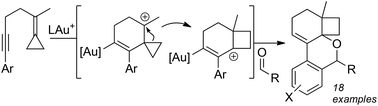
A diastereoselective, gold-catalyzed cascading cycloisomerization of alkylidene cyclopropane bearing 1,5-enynes that terminates in a cyclo-addition of aldehydes has been developed. This diastereoselective reaction provides convergent access to novel polycyclic molecular structures (18 examples), and tolerates a diverse scope of aldehydes. Mechanistic studies reveal that the catalytic cycle rests at a digold off-cycle intermediate, one of which was isolated.
- This article is part of the themed collection: Polycyclic Reactions in Synthesis and Biosynthesis

 Please wait while we load your content...
Please wait while we load your content...