Acid-catalysed intramolecular addition of β-ketoesters to 1,3-dienes†
Abstract
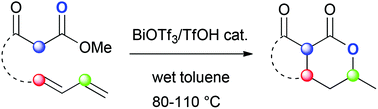
1,3-Dienyl β-keto esters are cyclised into bicyclolactones using the Bi(OTf)3/TfOH catalytic system. This reaction represents a rare case of simultaneous C–C and C–O bond formation at positions 1 and 3 of a 1,3-diene. Application to the synthesis of ramulosin is presented.


 Please wait while we load your content...
Please wait while we load your content...