Phosphorothioate anti-sense oligonucleotides: the kinetics and mechanism of the sulfurisation of phosphites by phenylacetyl disulfide (PADS)†
Abstract
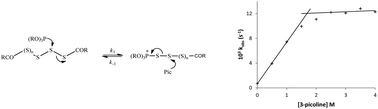
In the pharmaceutical industry the sulfurisation of nucleotide-phosphites to produce more biologically stable thiophosphates is often achieved using ‘aged’ solutions of phenylacetyl disulfide (PADS) which consist of a mixture of polysulfides that are more efficient sulfur transfer reagents. However, both ‘fresh’ and ‘aged’ solutions of PADS are capable of the sulfurisation of phosphites. The rates of both processes in acetonitrile are first order in sulfurising agent, phosphite and a pyridine base, although with ‘aged’ PADS the rate becomes independent of base at high concentrations. The Brönsted β values for sulfurisation using ‘fresh’ and ‘aged’ PADS with substituted pyridines are 0.43 and 0.26, respectively. With ‘fresh’ PADS the Brönsted βnuc = 0.51 for substituted trialkyl phosphites is consistent with a mechanism involving nucleophilic attack of the phosphite on the PADS disulfide bond to reversibly generate a phosphonium intermediate, the rate-limiting breakdown of which occurs by a base catalysed elimination process, confirmed by replacing the ionisable hydrogens in PADS with methyl groups. The comparable polysulfide phosphonium ion intermediate seen with ‘aged’ PADS presents a more facile pathway for product formation involving S–S bond fission as opposed to C–S bond fission.

 Please wait while we load your content...
Please wait while we load your content...