Transition-metal-free oxidative C5 C–H-halogenation of 8-aminoquinoline amides using sodium halides†
Abstract
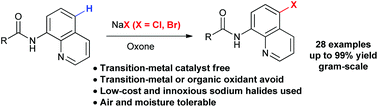
A simple and efficient transition-metal-free oxidative halogenation (Cl, Br) of the C5 C–H bond of 8-aminoquinoline amides has been developed. This method employed low-cost and innoxious sodium halides as halogenation reagents and oxone as an oxidant. The reactions demonstrated air and moisture tolerance, and good functional group compatibility, giving highly selective C5-halogenated products in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...