Enolate-mediated 1,3-dipolar cycloaddition reactions of allyl ketones with nitrile oxides: direct access to 3,5-disubstituted isoxazolines†
Abstract
TMG-promoted [3 + 2] organocatalytic 1,3-dipolar cycloaddition reactions of allyl ketones with in situ generated nitrile oxides have been developed. This strategy could generate 3,5-disubstituted isoxazolines in high yields and regioselectivities.
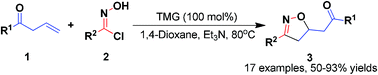

 Please wait while we load your content...
Please wait while we load your content...