Stereoselective construction of 2-vinyl 3-hydroxybenzopyran rings: total syntheses of teadenols A and B†
Abstract
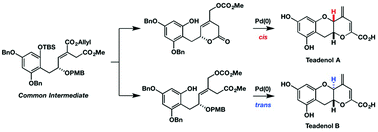
Total syntheses of teadenols A and B, isolated from fermented tea, were accomplished in a highly stereocontrolled manner. Key steps were an organocatalytic asymmetric α-aminoxylation reaction of an aldehyde and a palladium-catalyzed intramolecular allylic substitution with phenol. In the latter reaction, we utilized the different conformational preferences of cyclic and acyclic carbonate precursors to obtain cis- and trans-fused benzopyran rings, respectively, via intramolecular etherification.

 Please wait while we load your content...
Please wait while we load your content...