Chemoselective reduction of quinols as an alternative to Sonogashira coupling: synthesis of polysubstituted benzofurans†
Abstract
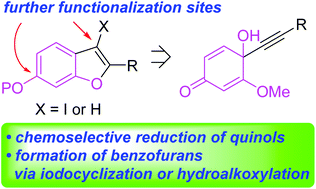
An efficient synthetic approach to polysubstituted benzofurans is described, using 2-methoxyquinone as a benzofuran backbone. Nucleophilic addition of terminal alkynes to 2-methoxy-1,4-benzoquinone afforded the corresponding quinols containing an alkyne unit, which were converted to phenols via mild Zn-mediated reduction. After proper protection of the free phenolic OH, 5-endo-dig iodocyclization allowed facile access to a number of 3-iodobenzofurans. In addition, it was demonstrated for the first time that o-methoxyarylalkynes underwent intramolecular hydroalkoxylation under the influence of AgOTf furnishing the corresponding benzofurans.

 Please wait while we load your content...
Please wait while we load your content...