Synthesis of 28a-homoselenolupanes and 28a-homoselenolupane saponins†
Abstract
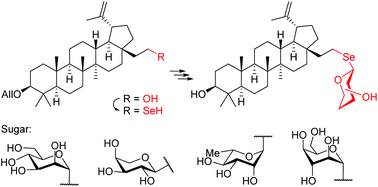
A practical synthesis of 28a-homo-28a-selenolupane triterpenes and the corresponding selenosaponins containing D-mannose, L-arabinose, L-rhamnose, and D-idose moieties is described. Selenium containing triterpenes were obtained from the readily available 3-O-allyl-homobetulin mesylate by nucleophilic substitution with the selenocyanate ion which upon reduction of the –SeCN group afforded the free selenol. Glycosylation using classical Schmidt donors gave 1,2-trans selenosaponins as the main product as well as minute amounts of 1,2-cis isomers. This is one of the very few examples of the synthesis of selenoglycosides by direct glycosylation of free selenols. The studied selenol showed high resistance to air oxidation resulting in good stability during the synthesis of selenolupane derivatives. Cytotoxic activities of new homoselenolupane derivatives were also evaluated in vitro and revealed that some triterpenes exhibited an interesting profile against human cancer cell lines.

 Please wait while we load your content...
Please wait while we load your content...