Rhodium(iii)-catalyzed ortho-alkenylation using a cyclic N-phosphoryl ketimine as the directing group†
Abstract
A novel cyclic N-phosphoryl ketimine structure can efficiently react with olefins as useful directing groups to construct a myriad of phosphate scaffolds via rhodium(III)-catalyzed ortho-alkenylation. This method provides a probability that the coupling products could be used as a building block to access more complex organic phosphorus compounds.
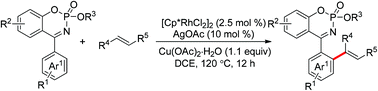

 Please wait while we load your content...
Please wait while we load your content...