A theoretical study of the Duff reaction: insights into its selectivity†
Abstract
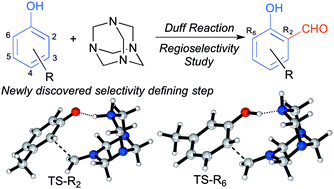
The Duff reaction is one of the most employed methods for the ortho-formylation of phenols; however, not much is truly known about its mechanism. Using DFT calculations, we disclose the first theoretical study regarding the selectivity-determining step of the reaction. We have found that this stage is governed by a hydrogen bond, that gives rise to a cyclohexa-2,4-dienone intermediate and establishes the position where the formylation will take place. These findings were evaluated by analysis of the reaction outcome of several non-symmetrically substituted phenols.

 Please wait while we load your content...
Please wait while we load your content...