Enantioselective synthesis of α,α-disubstituted α-amino acids via direct catalytic asymmetric addition of acetonitrile to α-iminoesters†
Abstract
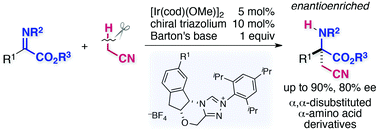
α,α-Disubstituted α-amino acids have attracted increasing interest due to their potential utility as building blocks of unnatural peptides. Herein we document an enantioselective entry to this class of compounds through the direct catalytic addition of acetonitrile to α-iminoesters bearing an N-thiophosphinoyl group. Chiral N-heterocyclic carbene complexes of [Ir(cod)(OMe)]2 catalytically rendered the catalytic generation of α-cyanocarbanions from acetonitrile in combination with Barton's base, followed by enantioselective addition to the imino carbonyl group, delivering a variety of enantioenriched α-cyanomethylated α,α-disubstituted α-amino acid derivatives.

 Please wait while we load your content...
Please wait while we load your content...