Highly regioselective synthesis of 4-tosylthiomorpholine via intramolecular cyclization of N-tethered thioalkenols†
Abstract
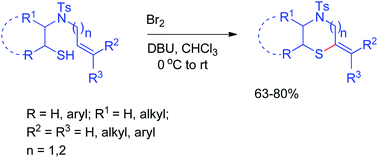
A one-pot, metal-free procedure has been developed for the synthesis of 4-tosylthiomorpholine from N-tethered thioalkenols via bromination, cyclization and subsequent elimination reaction in good yields. The reaction is found to be highly regioselective.

 Please wait while we load your content...
Please wait while we load your content...