I2-Mediated 2H-indazole synthesis via halogen-bond-assisted benzyl C–H functionalization†
Abstract
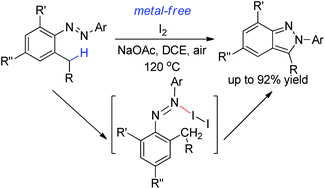
I2-Mediated benzyl C–H functionalization has been developed for the synthesis of 2H-indazoles, which features high efficiency, simple conditions and no need for metals. Mechanistic experiments and DFT calculations have revealed halogen bond assistance and a radical chain process for this reaction. The azo group and the bound iodine cooperate in the hydrogen abstraction step, which circumvents the thermodynamic disfavor of direct hydrogen abstraction by a simple iodine radical.

 Please wait while we load your content...
Please wait while we load your content...