Metal-free one-pot α-carboxylation of primary alcohols†
Abstract
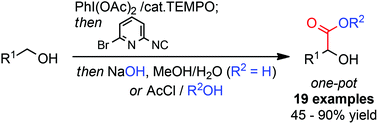
An efficient metal-free procedure for the formal α-carboxylation of primary alcohols has been developed. The method involves a one-pot oxidation/Passerini/hydrolysis sequence and provides access to α-hydroxy acids bearing a broad range of functional groups. A minor modification to the reaction conditions extends the range of accessible products to α-hydroxy esters.


 Please wait while we load your content...
Please wait while we load your content...