Dehydrative glycosylation with cyclic phosphonium anhydrides†
Abstract
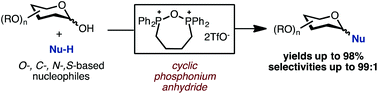
Cyclic phosphonium anhydrides generated from bis-phosphine oxides and trifluoromethanesulfonic anhydride are shown as general coupling reagents in a dehydrative glycosylation reaction of C1-hemiacetals. This reaction protocol is characterized by a broad substrate scope and high yields, including reactions of O-, C-, N-, and S-based nucleophiles with furanose, pyranose, and deoxysugar donors.


 Please wait while we load your content...
Please wait while we load your content...