(Biphenyl-2-alkyne) derivatives as common precursors for the synthesis of 9-iodo-10-organochalcogen-phenanthrenes and 9-organochalcogen-phenanthrenes†
Abstract
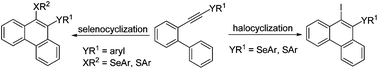
In this paper, we report our results on the cyclization of (biphenyl-2-alkyne) derivatives to give two different types of phenanthrene derivatives, 9-iodo-10-organochalcogen-phenanthrenes and 9-organochalcogen-phenanthrenes. The strategy for the synthesis was based on the use of electrophilic cyclization for the preparation of 9-iodo-10-organochalcogen-phenanthrenes and iron(III) chloride/diorganyl diselenide-mediated intramolecular cyclization to prepare 9-organochalcogen-phenanthrenes. The effects of solvent, temperature, reaction time and stoichiometry on the efficiency of cyclization reactions were investigated. The standard reaction conditions were compatible with many functional groups in the substrates, such as methyl, chlorine, fluorine and methoxyl. This protocol was efficient for diorganyl diselenides and disulfides but ineffective for diorganyl ditellurides. The resulting phenanthrenes were further functionalized through Sonogashira reactions followed by the electrophilic cyclization reaction to give the selenophene-fused aromatic compounds.


 Please wait while we load your content...
Please wait while we load your content...