A simple, modular synthesis of C4-substituted tryptophan derivatives†
Abstract
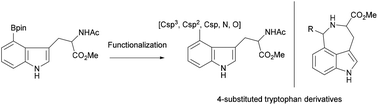
The modular and versatile synthesis of C4-substituted tryptophan derivatives by direct functionalization of easily available N-acetyl 4-boronate tryptophan methyl ester via transition metal-catalyzed and metal-mediated cross coupling reactions is described. The versatility of the chemistry is highlighted by the gram-scale synthesis of 4-boronated N-acetyl-tryptophan methyl ester and the rapid synthesis of C4-aryl, C4-alkyl, C4-cyano, C4-trifluoromethyl, C4-azido, and C4-hydroxy tryptophan derivatives. The utility of our methodology is illustrated through the quick approach to the tricyclic azepino indole skeleton embedded in many natural products.

 Please wait while we load your content...
Please wait while we load your content...