A solid-phase synthetic approach to pH-independent rhodamine-type fluorophores†
Abstract
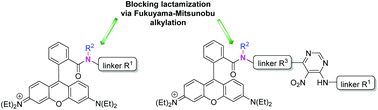
An efficient methodology using the Fukuyama–Mitsunobu reaction was successfully applied to prepare various Rhodamine B-based amides with the locked possibility to form a lactam ring. The procedure was developed for solid-phase synthesis, which can be advantageously applied to the synthesis of chemical libraries in a combinatorial fashion. A series of derivatives including aliphatic as well as aromatic rhodamine amides alkylated via a reaction with various alcohols were synthesized, and their spectral properties were investigated. Blocking lactamization via N-alkylation enabled us to prepare rhodamine derivatives with an excellent fluorescence response. In comparison to their non-alkylated counterparts, these derivatives exhibited pH independence and higher quantum yields.

 Please wait while we load your content...
Please wait while we load your content...