Carbocation–π interaction: evaluation of the stabilization by phenylalanine of a biochemical carbocation intermediate†
Abstract
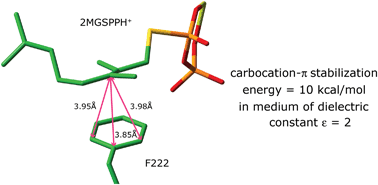
Computational analyses, using primarily density functional theory, have been used to determine the stabilization associated with the carbocation–π interaction of a biochemical carbocation intermediate binding to a phenylalanine residue in an enzyme active site. Studies of complexation between t-butyl cation and ethylbenzene, and of a model of a carbocation intermediate with a phenylalanine in the active site of geranyl diphosphate C-methyl transferase, have afforded the first quantitative evaluation of the stabilization that can be provided to a carbocation by an aromatic residue in an enzymatic reaction. Describing the hydrophobic surrounding medium using a dielectric constant between ε = 2 and ε = 4, the calculated carbocation–π stabilization energy lies in the range of 10–7.5 kcal mol−1.

 Please wait while we load your content...
Please wait while we load your content...