Synthesis and photophysics of extended π-conjugated systems of substituted 10-aryl-pyrenoimidazoles†
Abstract
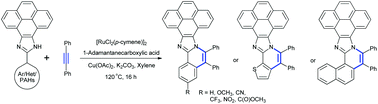
The synthesis of π-extended 10-aryl-pyrenoimidazoles having different substituents was realised via Ru(II)-catalyzed oxidative annulation of 10-aryl-pyrenoimidazole with diphenylacetylene. The single crystal X-ray structure of trifluoromethyl and carboxylate substituted annulated-10-aryl-pyrenoimidazoles confirms the near coplanarity of the pyrene and imidazole moieties and the presence of twisted conformation resulting in intermolecular C–H⋯π interactions. The lowest energy absorption maximum becomes red-shifted characteristic to the nature of the substituent owing to the extended π-conjugation, and specifically the nitro substituent shows intense absorption in the visible region with the maximum at 440 nm. All the molecules were found to show intense fluorescence both in solution and solid states. Strikingly, 170 nm red-shifted fluorescence with a large Stokes shift ca. 7000 cm−1 for the nitro derivative, a value nearly two-fold higher than the parent compound despite its rigid polyaromatic skeleton was observed. The combination of electron rich π-conjugated aromatic systems with electron deficient substituents induces the intramolecular charge transfer interactions, which has been corroborated with the theoretical calculations.

 Please wait while we load your content...
Please wait while we load your content...