Stereospecific prenylation of tryptophan by a cyanobacterial post-translational modification enzyme†
Abstract
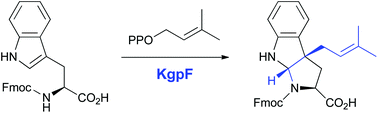
Prenylation is a key post-translational reaction to increase the structural diversity and bioactivity of peptides and proteins. Until now, only one post-translational modification enzyme, ComQ, has been identified to mediate the prenylation of a tryptophan residue in ribosomally synthesized peptides. Here, we report the in vitro characterization of KgpF, a novel prenyltransferase which transfers dimethylallyl moieties to tryptophan residues during kawaguchipeptin A biosynthesis. The stereospecific prenylation by KgpF was determined by a combination of in vitro dimethylallylation of Fmoc-tryptophan by KgpF and chemical synthesis of dimethylallylated Fmoc-tryptophan diastereomers. KgpF modified the tryptophan derivative with a dimethylallyl group at the 3 position of its indole ring, resulting in the formation of a tricyclic structure with the same scaffold as prenylation by ComQ, but with the opposite stereochemistry.

 Please wait while we load your content...
Please wait while we load your content...