Intramolecular oxyacetoxylation of N-allylamides: an expeditious synthesis of oxazolines and oxazines by using a PhI(OAc)2/hydrogen fluoride–pyridine system†
Abstract
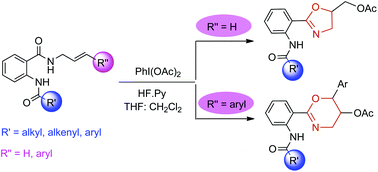
The synthesis of oxazolines and oxazines from N-allylamides was accomplished via an unprecedented reaction set. This reaction involved an intramolecular cyclization of N-allylamides resulting from nucleophilic attack of the allylamine on the benzoxazin-4-one. Unlike the previous literature, the isolable N-allylamide could readily be subjected to exo and endo fashion ring-closure under conditions to afford the title compounds.

 Please wait while we load your content...
Please wait while we load your content...