Structure–activity studies of non-steroid analogues structurally-related to neuroprotective estrogens†
Abstract
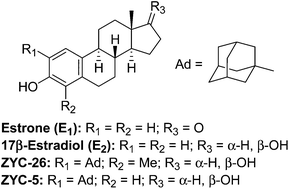
Estrone and 17β-estradiol are phenolic steroids that are known to be neuroprotective in multiple models of neuronal injury. Previous studies have identified the importance of their phenolic steroid A-ring for neuroprotection and have identified ortho substituents at the C-2 and C-4 positions on the phenol ring that enhance this activity. To investigate the importance of the steroid ring system for neuroprotective activity, phenolic compounds having the cyclopent[b]anthracene, cyclopenta[b]phenanthrene, benz[f]indene, benz[e]indene, indenes linked to a phenol, and a phenolic spiro ring system were prepared. New synthetic methods were developed to make some of the cyclopent[b]anthracene analogues as well as the spiro ring system. Compounds were evaluated for their ability to protect HT-22 hippocampal neurons from glutamate neurotoxicity and their activity relative to a potent neuroprotective analogue of 17β-estradiol was determined. An adamantyl substituent placed ortho to the phenolic hydroxyl group gave neuroprotective analogues in all ring systems studied.

 Please wait while we load your content...
Please wait while we load your content...