Screening of Neu5Acα(2–6)gal isomer preferences of siglecs with a sialic acid microarray†
Abstract
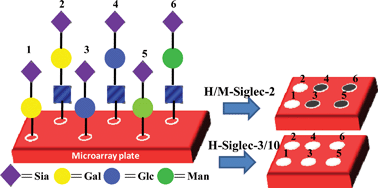
Sialic acids (Sias) are important terminal sugars on cell surfaces involved in a wide range of protein–carbohydrate interactions. Hence, agents modulating sias-mediated protein interactions are promising inhibitors or vaccine candidates. Here, we report the synthesis of Neu5Acα(2–6)Gal structural analogs and their binding to a series of siglecs. The results showed distinct binding patterns with conserved siglecs (hCD22 and mCD22) compared to rapid evolving siglecs (Siglecs -3 & -10).

 Please wait while we load your content...
Please wait while we load your content...