Highly enantioselective asymmetric Henry reaction catalyzed by novel chiral phase transfer catalysts derived from cinchona alkaloids†
Abstract
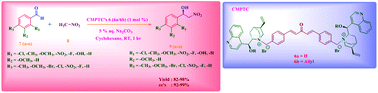
A new type of di-site chiral phase transfer catalyst has been designed and synthesized from cinchona alkaloids as a chiral precursor. The prepared catalysts are applied in the asymmetric Henry reaction to a wide range of aldehydes using mild concentrations of a base and solvent and under room-temperature conditions. Under the optimized reaction conditions, the highest chemical yields up to 99% along with an excellent enantiomeric excess (ee) up to 99% were obtained using the prepared cinchona alkaloid based chiral phase transfer catalysts.

 Please wait while we load your content...
Please wait while we load your content...