Transition metal-free N-arylation of secondary amides through iodonium salts as aryne precursors†
Abstract
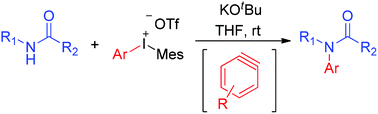
By using a diaryliodonium salt as a benzyne precursor, a transition metal-free approach for N-arylation of secondary amides is developed. This novel benzyne precursor, which can be prepared easily by a one step process from an aryl iodide, shows different reactivities with previous benzyne precursors in the N-arylation reaction. Mechanistic studies confirm the involvement of benzyne species (generated in situ from the diaryliodonium salts) as key intermediates.

 Please wait while we load your content...
Please wait while we load your content...