A halogen bond does not dictate the conformational preferences of cis-1,3-disubstituted cyclohexanes†
Abstract
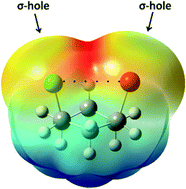
Halogen bonds are defined as interactions between halogens and a Lewis base in which the halogen (X) acts as the electrophilic species, and is typically explained by the presence of a σ-hole at the end of the C–X bond. Despite the important role of the halogen bond in intermolecularly interacting species, e.g. in acid–base reactions, enzyme inhibition and the supramolecular architecture, this interaction was not found to control the conformational equilibrium of some small model molecules, namely cis-1,3-disubstituted cyclohexanes. In addition, the attraction between the electrophilic (σ-hole) and nucleophilic regions is used to explain that the halogen bond was weaker than that in the species with parallel C–X bonds. Therefore, intramolecular halogen bonds should be used with caution to explain the conformational stability of organic compounds.

 Please wait while we load your content...
Please wait while we load your content...