Isothiourea-catalysed enantioselective pyrrolizine synthesis: synthetic and computational studies†
Abstract
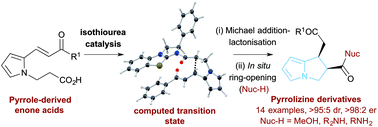
The catalytic enantioselective synthesis of a range of cis-pyrrolizine carboxylate derivatives with outstanding stereocontrol (14 examples, >95 : 5 dr, >98 : 2 er) through an isothiourea-catalyzed intramolecular Michael addition-lactonisation and ring-opening approach from the corresponding enone acid is reported. An optimised and straightforward three-step synthetic route to the enone acid starting materials from readily available pyrrole-2-carboxaldehydes is delineated, with benzotetramisole (5 mol%) proving the optimal catalyst for the enantioselective process. Ring-opening of the pyrrolizine dihydropyranone products with either MeOH or a range of amines leads to the desired products in excellent yield and enantioselectivity. Computation has been used to probe the factors leading to high stereocontrol, with the formation of the observed cis-steroisomer predicted to be kinetically and thermodynamically favoured.
- This article is part of the themed collection: Celebrating our 2018 prize and award winners

 Please wait while we load your content...
Please wait while we load your content...