Direct access to 2-amino-5-azidomethylfurans through palladium-catalyzed azidative cycloisomerization of homoallenyl amides†
Abstract
A room temperature, efficient, and palladium-catalyzed azidative cycloisomerization of homoallenyl amides using TMSN3 as the azidation reagent and PhI(O2CCF3)2 as the oxidant is described, producing a variety of 2-amino-5-azidomethylfurans in moderate to excellent yields. The products can be applied to the concise synthesis of 1,2,3-triazoles via a Cu-catalyzed azide–alkyne cycloaddition.
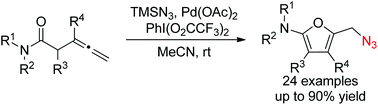

 Please wait while we load your content...
Please wait while we load your content...