Progress in aminosugar derived asymmetric organocatalysis
Abstract
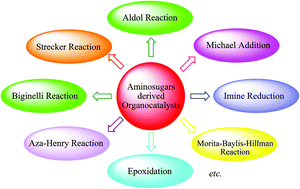
In the last decade aminosugars, especially D-glucoamine based organocatalysts, have been applied to catalyze various asymmetric reactions such as aldol reactions, Michael addition, Strecker reactions, Biginelli reactions, epoxidation, fluorination, and imine reduction, and for the synthesis of various biologically important molecules such as 3-alkylnitro-2-hydroxynaphthoquinones, trans-dihydrobenzofurans etc. Immense growth has been also observed in the structural modification of aminosugar based organocatalysts to obtain the best results from them. This review sheds light on such organocatalytic transformations reported in last the decade including the effect of the structural modification of sugar amines on their catalytic efficiency and the stereoselectivity of the reaction.

 Please wait while we load your content...
Please wait while we load your content...