A facile approach for the synthesis of C13–C24 fragments of maltepolides A, C and D†
Abstract
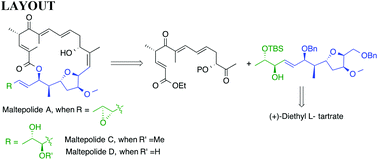
A linear, chiron approach for the synthesis of C13–C24 fragments of cytostatic maltepolides A, C and D consisting of a tetrahydrofuran subunit and a chiral alkenyl/alkyl substituent is achieved from (+)-diethyl L-tartrate. The other chiral stereocenters were generated by employing key reactions such as Crimmins aldol, alkynylation and CeCl3·7H2O mediated Luche reduction reactions.

 Please wait while we load your content...
Please wait while we load your content...