Copper–TEMPO-catalyzed synthesis of α-ketoamides via tandem sp3C–H aerobic oxidation and amination of phenethyl alcohol derivatives†
Abstract
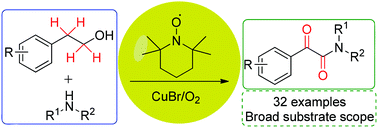
An efficient copper–TEMPO-catalyzed one-pot synthesis of α-ketoamides from phenethyl alcohol derivatives was developed firstly. Moreover, molecular oxygen in open air was employed as the oxidant with a broad substrate scope, which makes this methodology more practical. Based on some control experiments, a plausible mechanism was proposed.


 Please wait while we load your content...
Please wait while we load your content...