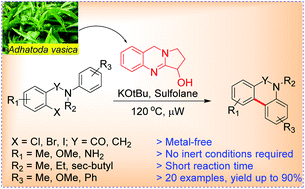
Microwave assisted synthesis of phenanthridinones and dihydrophenanthridines by vasicine/KOtBu promoted intramolecular C–H arylation†‡
Abstract
A simple, efficient, rapid and transition metal-free methodology has been developed by utilizing vasicine (a natural product), as a catalyst for the synthesis of phenanthridinones and dihydrophenanthridines. The reaction proceeds through intramolecular C–H arylation with aryl halides in the presence of KOtBu as a base under microwave irradiation in sulfolane as a solvent. The reaction proceeds well with various aryl iodides, bromides and more remarkably with less reactive aryl chlorides for 15 minutes, providing the corresponding products in 45–90% yields.

 Please wait while we load your content...
Please wait while we load your content...