Self-assembly of Ni-NTA-modified β-annulus peptides into artificial viral capsids and encapsulation of His-tagged proteins
Abstract
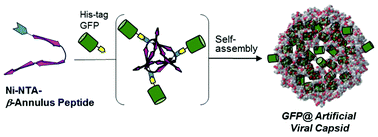
β-Annulus peptides bearing Cys at the N-terminal from tomato bushy stunt virus were synthesised using a standard Fmoc-protected solid-phase method, and the peptide was modified with Ni-NTA at the N-terminal. The Ni-NTA-modified β-annulus peptide self-assembled into virus-like nanocapsules of approximately 40 nm in diameter. The critical aggregation concentration of these nanocapsules in 10 mM Tris-HCl buffer (pH 7.3) at 25 °C was 0.053 μM, which is 470 times lower than that of unmodified β-annulus peptides. Moreover, size exclusion chromatography of the peptide assembly indicated encapsulation of His-tagged green fluorescent protein in the Ni-NTA-modified artificial viral capsid.

 Please wait while we load your content...
Please wait while we load your content...