BF3·OEt2-mediated syn-selective Meyer–Schuster rearrangement of phenoxy propargyl alcohols for Z-β-aryl-α,β-unsaturated esters†
Abstract
Synthesis of Z-β-aryl-α,β-unsaturated esters from readily available 1-aryl-3-phenoxy propargyl alcohols is achieved via a BF3-mediated syn-selective Meyer–Schuster rearrangement under ambient conditions. The reaction mechanism is postulated to involve an electrophilic borylation of an allene intermediate as the key step to kinetically control the stereoselectivity.
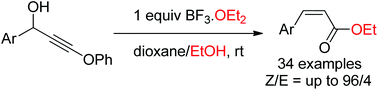

 Please wait while we load your content...
Please wait while we load your content...