Organocatalytic azomethine imine-olefin click reaction: high-yielding stereoselective synthesis of spiroindane-1,3-dione-pyrazolidinones†
Abstract
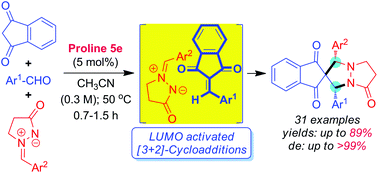
In search of developing new useful “click reactions”, herein we report the organocatalytic azomethine imine-olefin [3 + 2]-cycloaddition as a new click reaction for the synthesis of drug-like spiroindane-1,3-dione-pyrazolidinones from indane-1,3-diones, aldehydes and N,N-cyclic azomethine imines through amino acid-catalysis. The scope of this new click reaction is demonstrated using many examples with high reactivity, selectivity and yields.

 Please wait while we load your content...
Please wait while we load your content...