Iron-mediated oxidative C–H coupling of arenes and alkenes directed by sulfur: an expedient route to dihydrobenzofurans†
Abstract
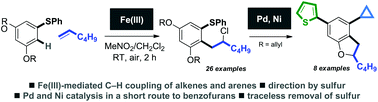
A novel route to medicinally-relevant dihydrobenzofurans utilises a sulfur-directed C–H ortho-coupling of arenes and unactivated terminal alkenes mediated by iron, and a palladium-catalysed deallylation/heterocyclisation sequence. The iron-mediated coupling affords linear products of alkene chloroarylation in good yield and with complete regioselectivity. The coupling likely proceeds by redox-activation of the arene partner by iron(III) and alkene addition to the resultant radical cation.
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery

 Please wait while we load your content...
Please wait while we load your content...