A DFT study on NHC-catalyzed intramolecular aldehyde–ketone crossed-benzoin reaction: mechanism, regioselectivity, stereoselectivity, and role of NHC†
Abstract
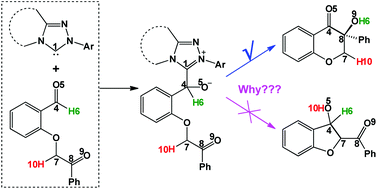
A systematic theoretical study has been carried out to understand the mechanism and stereoselectivity of N-heterocyclic carbene (NHC)-catalyzed intramolecular crossed-benzoin reaction of enolizable keto-aldehyde using density functional theory (DFT) calculations. The calculated results reveal that the most favorable pathway contains four steps, i.e., the nucleophilic attack of NHC on the carbonyl carbon atom of a formyl group, the formation of a Breslow intermediate, a ring-closure process coupled with proton transfer, and regeneration of the catalyst. For the formation of the Breslow intermediate via the [1,2]-proton transfer process, apart from the direct proton transfer mechanism, the base Et3N and the in situ generated Brønsted acid Et3N·H+ mediated proton transfer mechanisms have also been investigated; the free energy barriers for the crucial proton transfer steps are found to be significantly lowered by explicit inclusion of the Brønsted acid Et3N·H+. The computational results show that the ring-closure process is the stereoselectivity-determining step, in which two chirality centers assigned on the coupling carbon atoms are formed, and the S-configured diastereomer is the predominant product, which is in good agreement with the experimental observations. NCI and NBO analyses are employed to disclose the origin of stereoselectivity and regioselectivity. Moreover, a global reaction index (GRI) analysis has been performed to confirm that NHC mainly plays the role of a Lewis base. The mechanistic insights obtained in the present study should be valuable for the rational design of an effective organocatalyst for this kind of reaction with high stereoselectivity and regioselectivity.

 Please wait while we load your content...
Please wait while we load your content...