From glycals to aminosugars: a challenging test for new stereoselective aminohydroxylation and related methodologies
Abstract
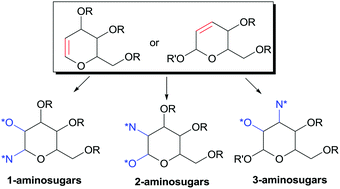
The introduction of amino functionalities in a regio- and stereoselective manner onto sugar scaffolds represents a great challenge in carbohydrate synthesis. The most relevant methods to access 1-, 2-, 3-amino or 1,2-diaminosugars starting from glycals and 2,3-hexenopyranosides derived from them are concisely reviewed. The main synthetic strategies for accessing this class of compounds are classified in intermolecular and intramolecular approaches and the key features of each class are discussed. This review highlights how carbohydrate derivatives always pose great challenges representing a benchmark for assessing the efficiency of stereoselective strategies, and aims to give the readers inspiration for the development of new procedures.

 Please wait while we load your content...
Please wait while we load your content...