Design, synthesis, and in vitro evaluation of a fluorescently labeled irreversible inhibitor of the catalytic subunit of cAMP-dependent protein kinase (PKACα)†
Abstract
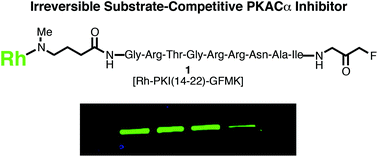
The design and development of irreversible kinase inhibitors is an expanding frontier of kinase drug discovery. The current approach to develop these inhibitors utilizes ATP-competitive inhibitor scaffolds to target non-catalytic cysteines in the kinase ATP-binding site. However, this approach is limited as not all kinases have a cysteine in the ATP-binding site that can be targeted. In this work, we report a complementary approach to developing irreversible kinase inhibitors that utilizes the substrate-binding site. Using the catalytic subunit of cAMP-dependent protein kinase (PKACα) as a model system, we have designed and synthesized an irreversible inhibitor based on the substrate-competitive inhibitor scaffold PKI(14-22) that covalently modifies non-catalytic Cys199 in the PKACα substrate-binding site. The new compound inhibits PKACα (IC50 = 11.8 ± 1.1 nM), is ∼100-fold selective for PKACα in a kinase panel, and covalently labels the kinase as demonstrated by fluorescence, mass spectrometry, and kinetics experiments. This study demonstrates the feasibility of utilizing this new approach to develop irreversible inhibitors for any of the eighty-nine kinases that possess a similar non-catalytic cysteine in their substrate-binding sites.

 Please wait while we load your content...
Please wait while we load your content...