Hydrohalogenative aromatization of multiynes promoted by ruthenium alkylidene complexes†
Abstract
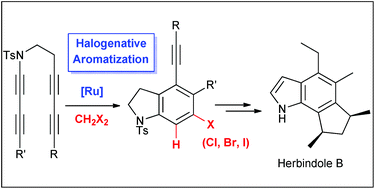
A new functionalization method of arynes promoted by a novel catalytic role of the Grubbs-type ruthenium alkylidene complex is described. Through a sequence of aryne formation followed by their halo-functionalization, various bis-1,3-diynes were directly converted to functionalized aryl chlorides, bromides and iodides in good yields in the presence of a catalytic amount of a ruthenium alkylidene complex and halogenated hydrocarbons such as CH2Cl2, CHCl3, CH2Br2, and CH2I2. The utility of this novel transformation is demonstrated by a formal synthesis of herbindole B.
 Please wait while we load your content...
Please wait while we load your content...