Synthesis and determination of absolute configuration of a non-peptidic αvβ6 integrin antagonist for the treatment of idiopathic pulmonary fibrosis†
Abstract
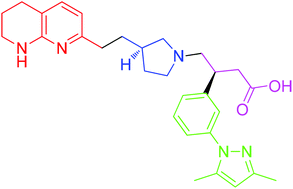
A diastereoselective synthesis of (S)-3-(3-(3,5-dimethyl-1H-pyrazol-1-yl)phenyl)-4-((R)-3-(2-(5,6,7,8-tetrahydro-1,8-naphthyridin-2-yl)ethyl)pyrrolidin-1-yl)butanoic acid (1), a potential therapeutic agent for the treatment of Idiopathic Pulmonary Fibrosis, which is currently undergoing Phase I clinical trials is reported. The key steps in the synthesis involved alkylation of 2-methylnaphthyridine with (R)-N-Boc-3-(iodomethyl)-pyrrolidine, and an asymmetric Rh-catalysed addition of an arylboronic acid to a 4-(N-pyrrolidinyl)crotonate ester. The overall yield of the seven linear step synthesis was 8% and the product was obtained in >99.5% ee proceeding with 80% de. The absolute configuration of 1 was established by an alternative asymmetric synthesis involving alkylation of an arylacetic acid using Evans oxazolidinone chemistry, acylation using the resulting 2-arylsuccinic acid, and reduction. The absolute configuration of the benzylic asymmetric centre was established as (S).

 Please wait while we load your content...
Please wait while we load your content...